Author:XLIFESC
Release date:2022-06-10
The 2022 Annual Meeting of the American Society of Clinical Oncology (ASCO) is currently being held in Chicago. As the world's most authoritative and academically highest-level clinical oncology conference, ASCO showcases the latest international advances in oncology basic research and clinical frontier research to oncology researchers worldwide. This year's ASCO conference features 15 cancer research studies conducted in China selected for abstract oral presentations. Among them, XlifeSc TCR-T cell immunotherapy asset TAEST16001 is the only project completed Phase I clinical trials in China and selected for oral presentation, with its clinical research results recognized by ASCO.
On the morning of June 5, 2022, Beijing time, at the sarcoma session of the ASCO annual meeting, Professor Zhang Xing from the Sun Yat-sen University Cancer Center delivered the oral presentation, revealing the Phase I clinical research data of TAEST16001, the first TCR-T asset in China to receive an IND approval. The results showed an objective response rate (ORR) of 41.7%, with clinical safety and efficacy results comparable to those of the asset from GSK targeting the same antigen, gaining recognition from peers and attracting significant global industry attention!


Study Objectives and Population
A Phase I clinical study evaluating the safety, pharmacokinetics (PK), pharmacodynamics (PD), and efficacy of NY-ESO-1 tumor antigen-specific TCR-T cells (TAEST16001) in treating patients with advanced soft tissue sarcoma with HLA-A*02:01 genotype.
As of December 31, 2021, 12 patients with advanced soft tissue sarcoma were enrolled.
Safety Data
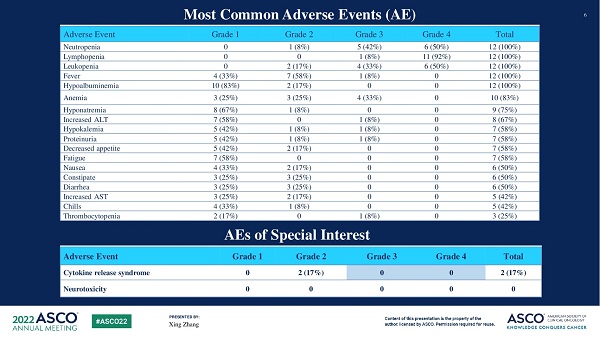
• TAEST16001 cells were well-tolerated with no dose-limiting toxicity observed.
• The most frequently reported Grade 3 adverse events included lymphopenia (n=12), leukopenia (n=10), neutropenia (n=11), anemia (n=4), thrombocytopenia (n=1), hypokalemia (n=1), and fever (n=1).
• Maximum tolerated dose (MTD) was not reached.
• Two patients experienced Grade 2 cytokine release syndrome (CRS) which resolved after symptomatic treatment. No patients experienced neurotoxicity or severe adverse events related to cell infusion.
Efficacy Data
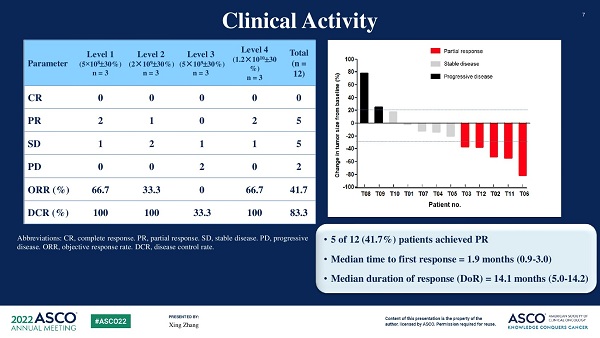
• Among the 12 evaluable patients, partial response (PR) was observed in 5 patients, stable disease (SD) in 5 patients, and progressive disease (PD) in 2 patients.
• The overall response rate was 41.7%.
• The median time to initial response was 1.9 months (range: 0.9 to 3.0), and the median duration of response was 14.1 months (range: 5.0 to 14.2).
Study Conclusion
TAEST16001 cell therapy demonstrated acceptable tolerability with no MTD reached, along with encouraging efficacy results. This data support further research of TAEST16001 cells in advanced soft tissue sarcoma.
About TAEST16001
TAEST16001 is independently developed by XlifeSc Technology (Guangdong) Co., Ltd. It is the first TCR-T cell immunotherapy asset in China to receive IND approval. Phase I clinical results indicate the asset is safe and effective, and Phase II clinical trials are set to commence in China.
About XlifeSc
XlifeSc Technology (Guangdong) Co., Ltd. (referred to as "XlifeSc") is a leading enterprise focused on the research and development of cell immunotherapy assets and technologies in the TCR field. The company's vision is to "solve human health challenges and set a benchmark for cancer treatment," with the mission to "focus on TCR, empower T cells, and conquer solid tumors."
XlifeSc possesses proprietary TCR core technology and has established a comprehensive TCR-T asset research and development technology system as well as a assetion and preparation system. The TCR-T asset R&D technology system includes: 1) an antigen peptide discovery platform, 2) a high-affinity TCR platform, and 3) a TCR-T development platform. The TCR-T assetion and preparation system includes: 1) an automated cell assetion platform and 2) a quality control platform. This has resulted in a complete innovation industry chain for TCR-T cell assets. The research and development technology system of XlifeSc has generated a rich pipeline of ongoing and reserve projects, covering proprietary targets related to solid tumors and assets that cover the HLA typing of the Chinese population, reaching an international leading level.
Currently, XlifeSc has two investigational assets that have received IND approvals in China. The first asset, TAEST16001, is indicated for soft tissue sarcoma and has completed Phase I clinical trials, with Phase II clinical trials about to commence. The second asset, TAEST1901, is indicated for primary liver cancer and is about to initiate Phase I clinical trials.